Matthew T. Eddy, Tsyr-yan Yu, Gerhard Wagner, Robert G. Griffin
Journal of Biomolecular NMR DOI: 10.1007/s10858-019-00242-8
The second isoform of the human voltage dependent anion channel (VDAC2) is a mitochondrial porin that translocates calcium and other metabolites across the outer mitochondrial membrane. VDAC2 has been implicated in cardioprotection and plays a critical role in a unique apoptotic pathway in tumor cells. Despite its medical importance, there have been few biophysical studies of VDAC2 in large part due to the difficulty of obtaining homogeneous preparations of the protein for spectroscopic characterization. Here we present high resolution magic angle spinning nuclear magnetic resonance (NMR) data obtained from homogeneous preparation of human VDAC2 in 2D crystalline lipid bilayers. The excellent resolution in the spectra permit several sequence-specific assignments of the signals for a large portion of the VDAC2 N-terminus and several other residues in two- and three-dimensional heteronuclear correlation experiments. The first 12 residues appear to be dynamic, are not visible in cross polarization experiments, and they are not sufficiently mobile on very fast timescales to be visible in 13C INEPT experiments. A comparison of the NMR spectra of VDAC2 and VDAC1 obtained from highly similar preparations demonstrates that the spectral quality, line shapes and peak dispersion exhibited by the two proteins are nearly identical. This suggests an overall similar dynamic behavior and conformational homogeneity, which is in contrast to two earlier reports that suggested an inherent conformational heterogeneity of VDAC2 in membranes. The current data suggest that the sample preparation and spectroscopic methods are likely applicable to studying other human membrane porins, including human VDAC3, which has not yet been structurally characterized
Yu-Min Kao, Chuan-Hao Cheng, Ming-Lun Syue, Hsin-Yu Huang, I-Chia Chen, Tsyr-Yan Yu*, and Li-Kang Chu*
J. Phys. Chem. B
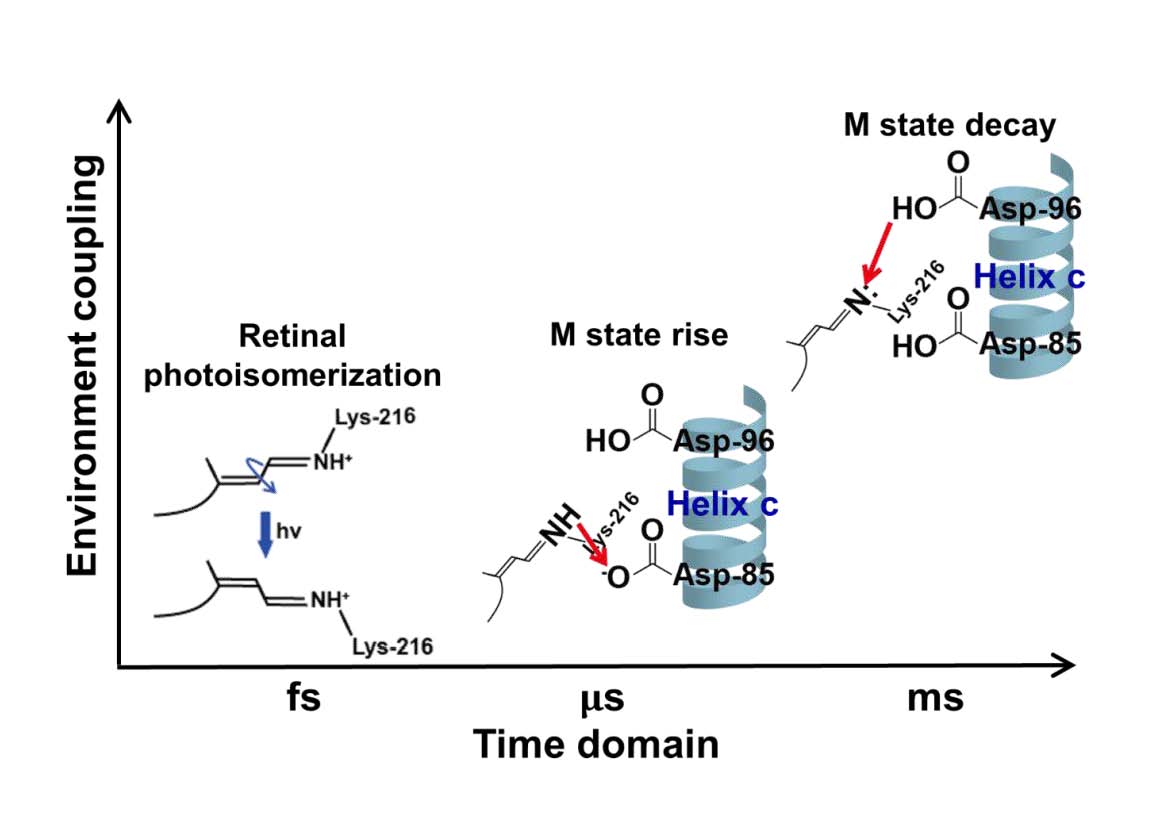
Preparing transmembrane protein in controllable lipid bilayers is essential for unravelling the coupling of the environments and its dynamic functions. Monomerized bacteriorhodopsin (mbR) embedded in covalently circularized nanodiscs was prepared with dimyristoyl phosphatidylglycerol (DMPG) lipid and circular membrane scaffold proteins of two different sizes, cE3D1 and cΔH5, respectively. The retinal photoisomerization kinetics and thermodynamic photocycle were examined by femtosecond and nanosecond transient absorption, respectively, covering the time scale from femtoseconds to hundreds of milliseconds. The kinetics of the retinal isomerization and proton migration from the protonated Schiff base to Asp-85 were not significantly different for monomeric bR solubilized in Triton X-100 or embedded in circularized nanodiscs. This can be ascribed to the local tertiary structures at the retinal pocket vicinity being similar among monomeric bR in various membrane mimicking environments. However, the aforementioned processes are intrinsically different for trimeric bR in purple membrane (PM) and delipidated PM. The reprotonation of the deprotonated Schiff base from Asp-96 in association with the decay of intermediate M, which involved wide-ranged structural alteration, manifested a difference in terms of the oligomeric statuses, as well as a slight dependence on the size of the nanodisc. In summary, bR oligomeric statuses, rather than the environmental factors, such as membrane mimicking systems and nanodisc size, play a significant role in bR photocycle associated with short-range processes, such as the retinal isomerization and deprotonation of protonated Schiff base at the retinal pocket. On the other hand, the environmental factors, such as the types of membrane mimicking systems and the size of nanodiscs, affect those dynamic processes involving wider structural alterations during the photocycle.
Vivien Yeh, Tsung-Yen Lee, Chung-Wen Chen, Pai-Chia Kuo, Jessie Shiue, Li-Kang Chu* & Tsyr-Yan Yu*
Scientific Reports, 8, 13501 (2018).
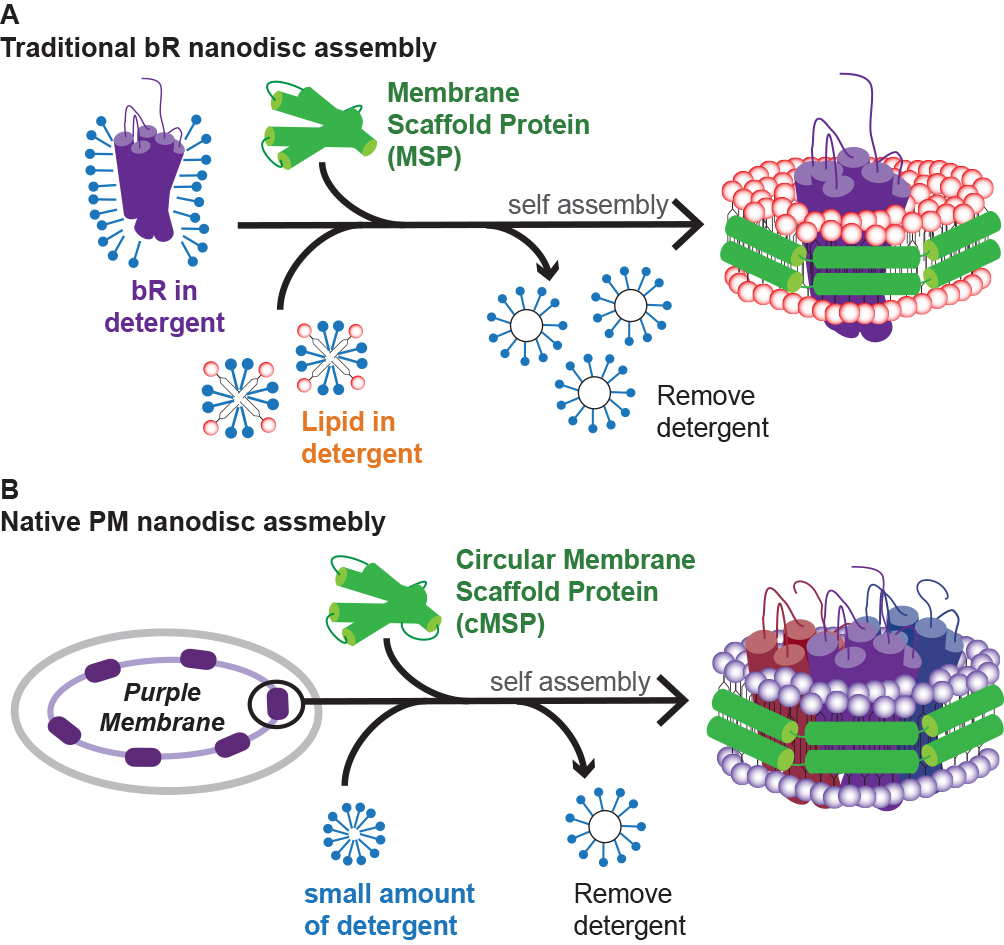
Incorporating membrane proteins into membrane mimicking systems is an essential process for biophysical studies and structure determination. Monodisperse lipid nanodiscs have been found to be a suitable tool, as they provide a near-native lipid bilayer environment. Recently, a covalently circularized nanodisc (cND) assembled with a membrane scaffold protein (MSP) in circular form, instead of conventional linear form, has emerged. Covalently circularized nanodiscs have been shown to have improved stability, however the optimal strategies for the incorporation of membrane proteins, as well as the physicochemical properties of the membrane protein embedded in the cND, have not been studied. Bacteriorhodopsin (bR) is a seven-transmembrane helix (7TM) membrane protein, and it forms a two dimensional crystal consisting of trimeric bR on the purple membrane of halophilic archea. Here it is reported that the bR trimer in its active form can be directly incorporated into a cND from its native purple membrane. Furthermore, the assembly conditions of the native purple membrane nanodisc (PMND) were optimized to achieve homogeneity and high yield using a high sodium chloride concentration. Additionally, the native PMND was demonstrated to have the ability to assemble over a range of different pHs, suggesting flexibility in the preparation conditions. The native PMND was then found to not only preserve the trimeric structure of bR and most of the native lipids in the PM, but also maintained the photocycle function of bR. This suggests a promising potential for assembling a cND with a 7TM membrane protein, extracted directly from its native membrane environment, while preserving the protein conformation and lipid composition.
Orion Shih, Yi-Qi Yeh, Kuei-Fen Liao, Chun-Jen Su, Pei-Hao Wu, Richard K. Heenan, Tsyr-Yan Yu*, and U-Ser Jeng*
Journal of Physical Chemistry Letters
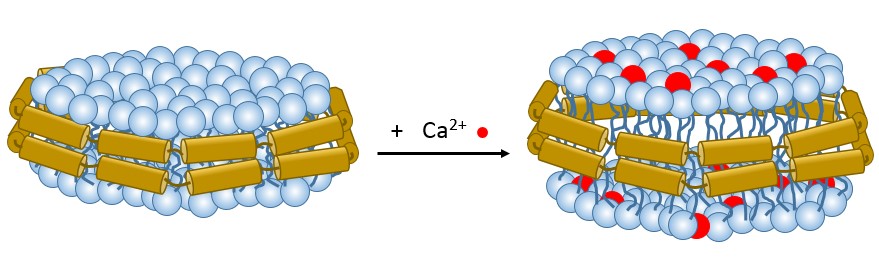
Direct binding of calcium ions (Ca2+) to phospholipid membranes is an unclarified yet critical signaling pathway in diverse Ca2+-regulated cellular phenomena. Here, high-pressure-liquid-chromatography, small-angle X-ray scattering (SAXS), UV-Vis absorption, and differential refractive index detections are integrated to probe Ca2+-binding to the zwitterionic lipid membranes in nanodiscs. The responses of the membranes upon Ca2+-binding, in composition and conformation, are quantified through integrated data analysis. The results indicate that Ca2+ binds specifically into the phospholipid head-group zone, resulting in membrane charging and membrane swelling, with a saturated Ca2+-lipid binding ratio of 1:8. A Ca2+-binding isotherm to the nanodisc is further established and yields an unexpectedly high binding constant K = 4260 M-1 and a leaflet potential of ca. 100 mV based on a modified Gouy-Chapman model. The calcium-lipid binding ratio, however, drops to 40% when the nanodisc undergoes a gel-to-fluid phase transition, leading to an effective charge capacity of a few mF/cm2.
Yustina yusuf, Julien Massiot, Yu-Ting Chang, Pei-Hao Wu, Vivien Yeh, Pai-Chia Kuo, Jessie Shiue*, and Tsyr-Yan Yu*
Langmuir 34, 3525−3532 (2018).
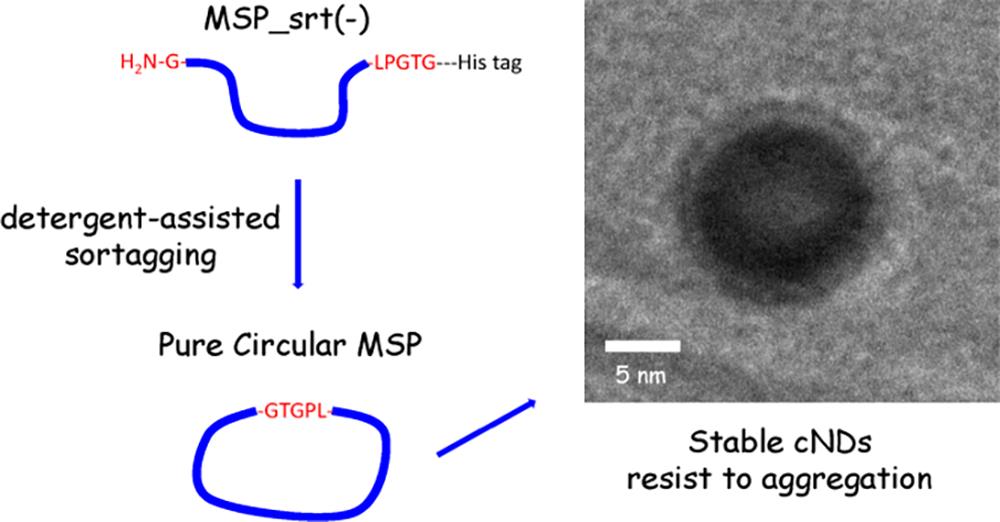
Lipid nanodiscs are widely used platforms for studying membrane proteins in a near-native environment. Lipid nanodiscs made with membrane scaffold proteins (MSPs) in linear form have been well studied. Recently, a new kind of nanodisc made with MSPs in circular form, referred to as covalently circularized nanodiscs (cND), and is reported to have some possible advantages in various applications. Given the potential of nanodisc technology, researchers in the field are very interested in learning more about this new kind of nanodisc, such as the reproducibility, the production yield, and the possible pros and cons of using it. However, research on these issues is lacking. Here we report a new study on nanodiscs made with circular MSPs, which are produced from a method different to the previously reported method. We show that our novel production method, detergent-assisted sortase-mediated ligation, can effectively avoid high-molecular weight byproducts, and also significantly improves the yield of the target proteins up to around eighty percent for larger circular MSP constructs. In terms of the application of circular MSPs, we demonstrate that they can be used to assemble nanodiscs using both synthetic lipids and native lipid extract as the source of lipids. We also show that bacteriorhodopsin (bR) can be successfully incorporated into this new kind of cND. Moreover, we found that cNDs have improved stability against both heat and high-concentration induced aggregations, making them more beneficial for related applications.
Tsung-Yen Lee, Vivien Yeh, Julia Chuang, Jerry Chun Chung Chan, Li-Kang Chu*, Tsyr-Yan Yu*
Biophys. J. 2015, 109(9), 1899-1906.
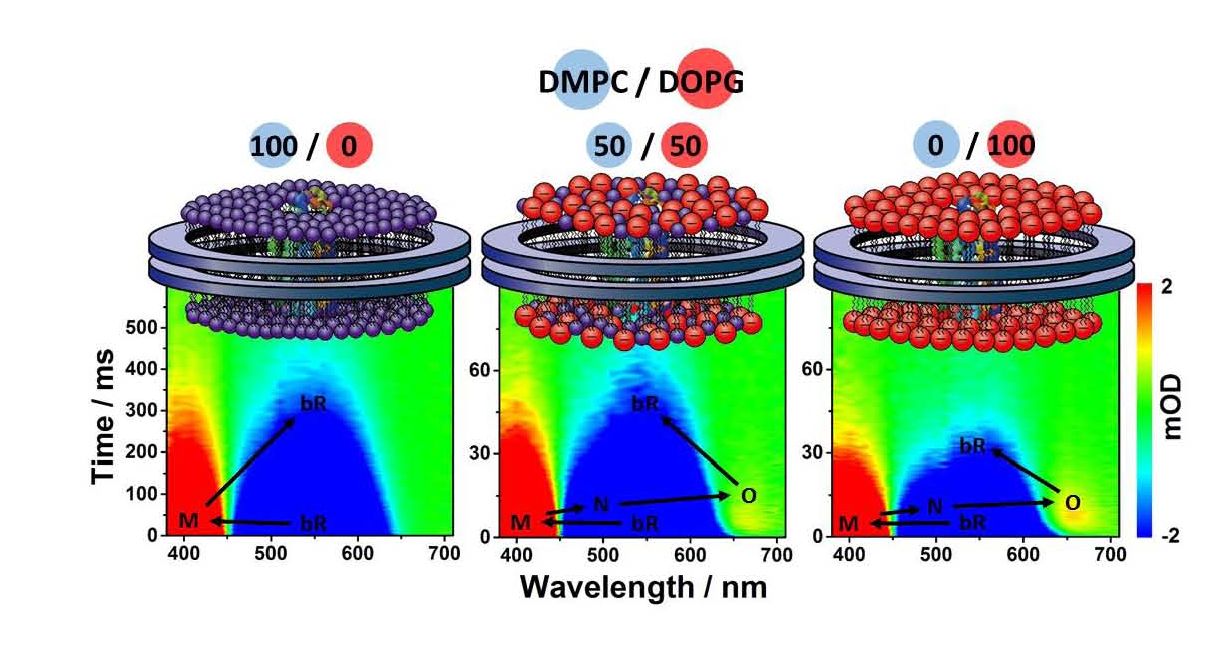
Monodisperse lipid nanodiscs are particularly suitable for characterizing membrane protein in near-native environment. To study the lipid-composition dependence of photocycle kinetics of bacteriorhodopsin (bR), transient absorption spectroscopy was utilized to monitor the evolution of the photocycle intermediates of bR reconstituted in nanodiscs composed of different ratios of the zwitterionic lipid (DMPC, dimyristoyl phosphatidylcholine; DOPC, dioleoyl phosphatidylcholine) to the negatively charged lipid (DOPG, dioleoyl phosphatidylglycerol; DMPG, dimyristoyl phosphatidylglycerol). The characterization of ion-exchange chromatography showed that the negative surface charge of nanodiscs increased as the content of DOPG or DMPG was increased. The steady-state absorption contours of the light-adapted monomeric bR in nanodiscs composed of different lipid ratios exhibited highly similar absorption features of the retinal moiety at 560 nm, referring to the conservation of the tertiary structure of bR in nanodiscs of different lipid compositions. In addition, transient absorption contours showed that the photocycle kinetics of bR was significantly retarded and the transient populations of intermediates N and O were decreased as the content of DMPG or DOPG was reduced. This observation could be attributed to the negatively charged lipid heads of DMPG and DOPG, exhibiting similar proton relay capability as the native phosphatidylglycerol (PG) analog lipids in the purple membrane. In this work, we not only demonstrated the usefulness of nanodiscs as a membrane-mimicking system, but also showed that the surrounding lipids play a crucial role in altering the biological functions, e.g., the ion translocation kinetics of the transmembrane proteins.
