Yen-Ju Wu, Kaito Takahashi, and Jim Jr-Min Lin
J. Phys. Chem. A, 127, 39, 8059–8072 (2023).
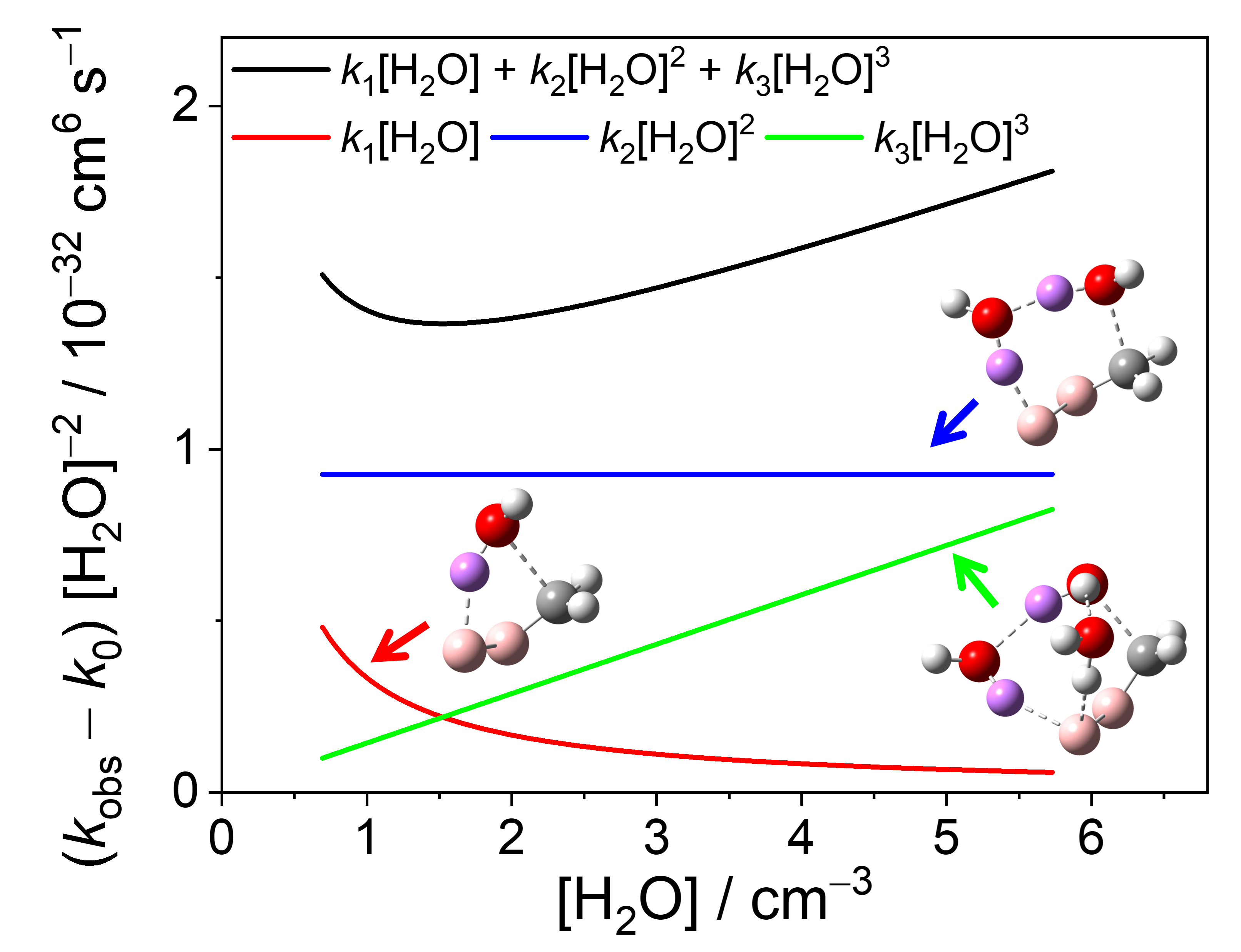
The kinetics of the simplest Criegee intermediate (CH2OO) reaction with water vapor was revisited. By improving signal-to-noise ratio and the precision of water concentration, we found that the kinetics of CH2OO at 298 K involves not only two water molecules but also one and three water molecules. Our experimental results suggest that the decay of CH2OO can be described as: d[CH2OO]/dt = − kobs[CH2OO]; kobs = k0 + k1[water] + k2[water]2 + k3[water]3; k1 = (3.87 ± 0.71) × 10−16 cm3 s−1, k2 = (11.07 ± 0.62) × 10−33 cm6 s−1, k3 = (1.39 ± 0.17) × 10−50 cm9 s−1 at 300 Torr with the respective Arrhenius activation energies of Ea1 = 2.6 ± 1.8 kcal mol−1, Ea2 = −10.8 ± 1.6 kcal mol−1, Ea3 = −18.0 ± 3.8 kcal mol−1. The contribution of the k3[water]3 term becomes insignificant at high temperatures around 345 K, but is not ignorable at room temperature and lower temperatures. By quantifying the concentrations of H2O and D2O with a Coriolis-type direct mass flow sensor, the kinetic isotope effect (KIE) was investigated at 300 Torr and KIE(k1) = k1(H2O)/k1(D2O) = 1.25 ± 0.32; similarly, KIE(k2) = 2.09 ± 0.37 and KIE(k3) = 1.03 ± 0.13. These mild KIE values are consistent with theoretical calculations based on transition state theory and high-level quantum chemistry methods. This confirms that the title reaction has a broad and low barrier and the reaction coordinate involves not only the motion of a hydrogen atom but also that of an oxygen atom. Comparing the results recorded under 300 Torr (N2 buffer gas) with those under 600 Torr, a weak pressure effect of k3 was found. From quantum chemistry calculations, we found that the CH2OO + 3H2O reaction is dominated by the reaction pathways involving a ring structure consisting of two water molecules which facilitate the hydrogen atom transfer while the third water molecule is hydrogen-bonded outside the ring. Furthrmore, analysis based on dipole capture rates showed that the CH2OO(H2O) + (H2O)2 and CH2OO(H2O)2 + H2O pathways will dominate in this three water reaction.
Kaito Takahashi
ChemPlusChem, 88, e202300354
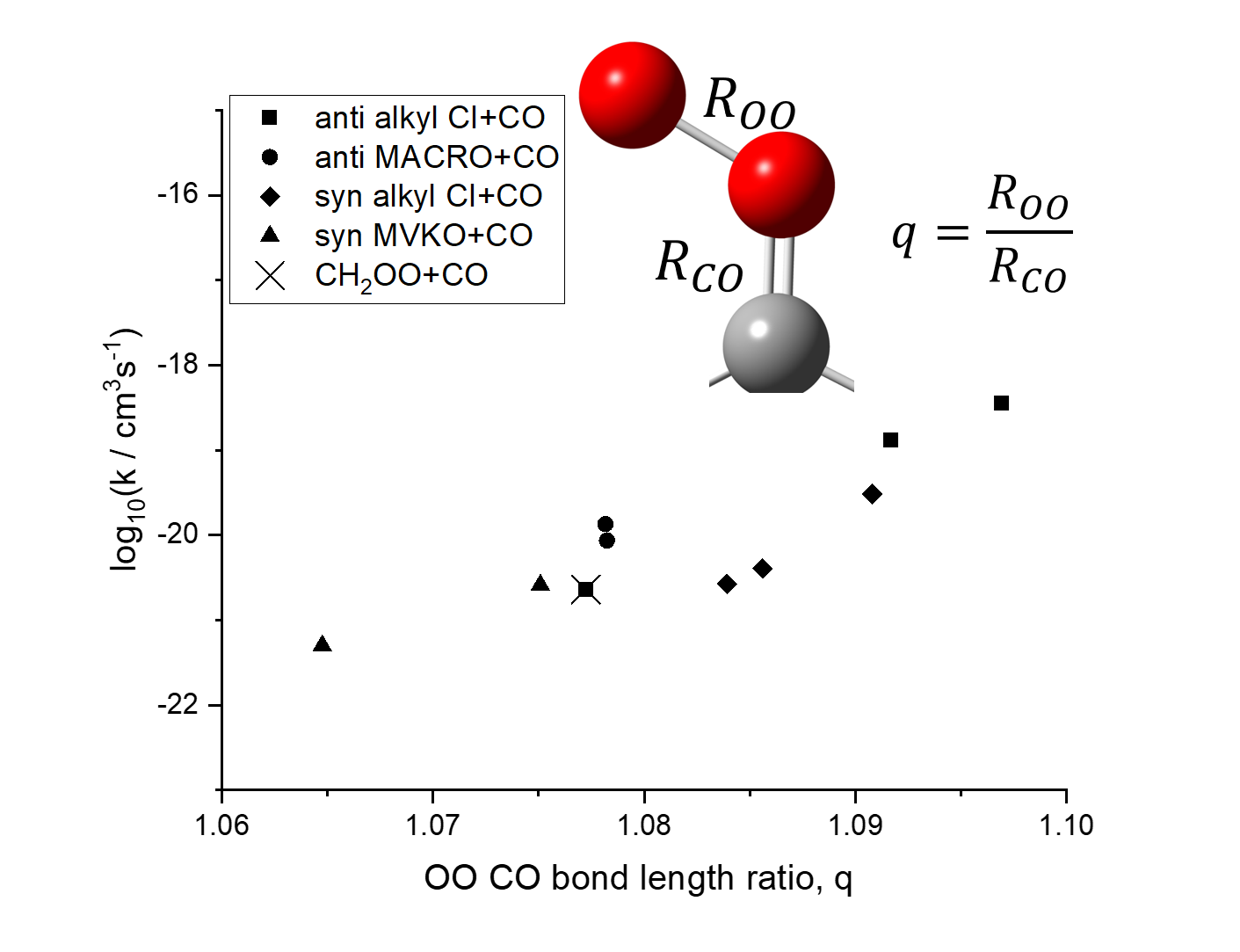
Criegee intermediates (CIs), R1R2COO, are active molecules produced in the atmosphere from the ozonolysis of alkenes. Here, we systematically evaluated the reactivity of ten CIs with carbon monoxide and carbon dioxide using CCSD(T)-F12/cc-pVTZ-F12//B3LYP/6-311+G(2d,2p) energies and transition state theory. Many previous studies focused on alkyl substitution, but here we evaluated both alkyl and vinyl substitution toward the reactivity by studying five anti-type CIs: CH2OO, anti-CH3CHOO, anti-cis-C2H5CHOO, anti-trans-MACRO, anti-cis-MACRO; and five syn-type CIs: syn-CH3CHOO, (CH3)2COO, syn-trans-C2H5CHOO, syn-trans-MVKO, and syn-cis-MVKO. Our study showed that reactions involving CO2 have a large substituent dependence varying nearly five orders of magnitude, while those involving CO have a much smaller two orders of magnitude difference. Analysis based on the strain interaction model showed that deformation of the CI is an important feature in determining the reactivity with CO2. On the other hand, we used the OO and CO bond ratios to analyze the zwitterionic character of the CIs. We found that vinyl substitution with π-conjugation results in smaller zwitterionic character and lower reactivity with CO. Lastly, the reactivity of CIs with CO as well as CO2 were found to be not fast enough to be important in an atmospheric context.
Klichchupong Dabsamut and Kaito Takahashi
Carbon, 218, 118672 (2024)
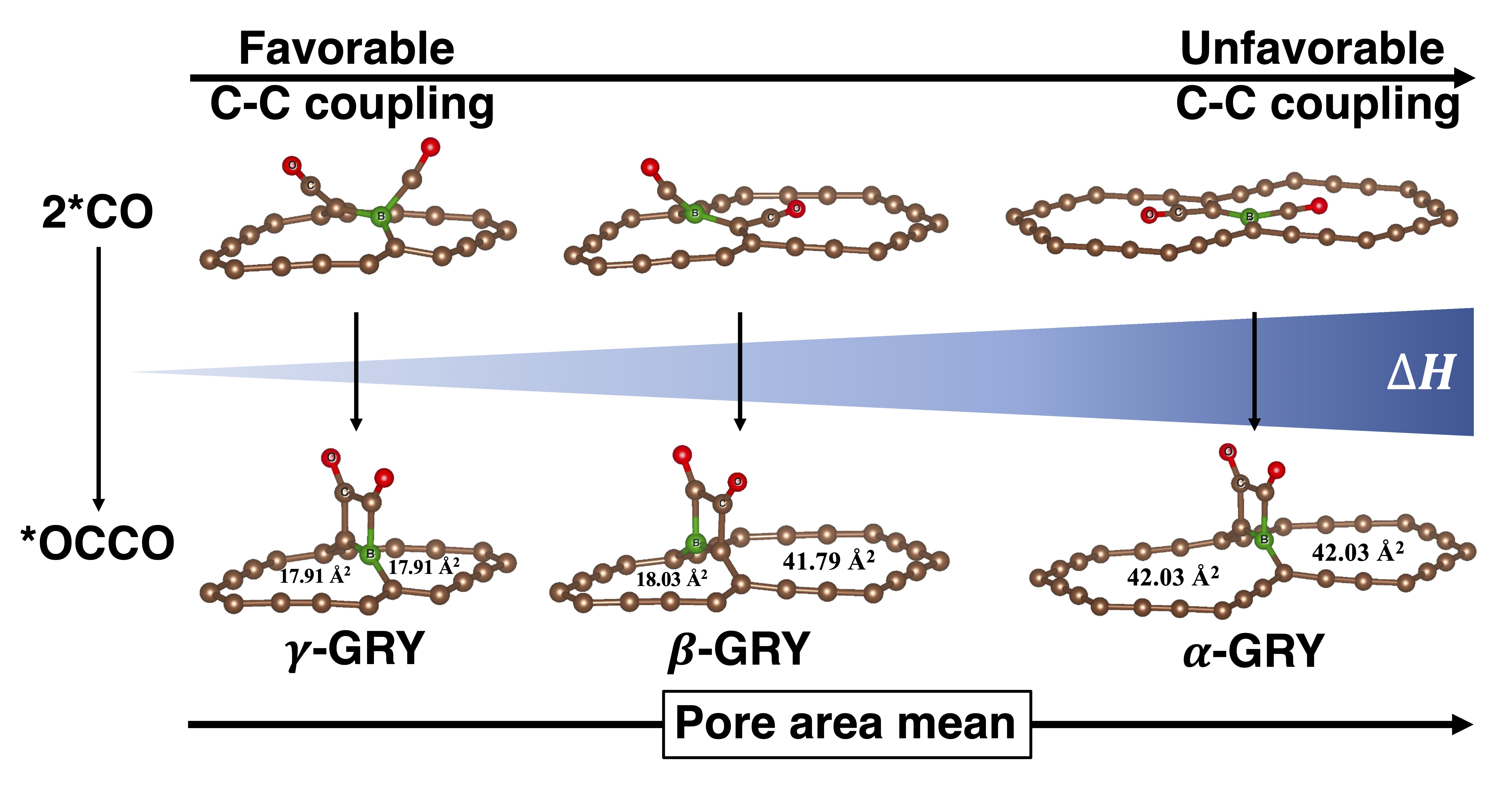
Graphyne is an interesting carbon allotrope characterized by sp-sp2 hybridized carbon bonds, which allow for structure variability. Though there are studies evaluating the electrical and mechanical properties of various structures, studies utilizing its structural variety toward catalyst applications are minor. In electrocatalytic CO2 reduction reactions (CRR), producing valuable C2+ products, such as C2H4 or C2H5OH, under mild conditions is still challenging. The bottleneck has been assigned to the carbon-carbon (C-C) coupling reaction, such as 2*CO → *OCCO. In this reaction, one requires strong binding to the catalyst to enhance neighboring CO concentration, but at the same time, one needs to have low C-C coupling barriers making the OC-CO bond. Following our previous study, which showed B-doped γ-graphyne can be a catalyst for ethanol production, we conducted theoretical investigations on a range of B-doped graphyne families using density functional theory. Out of the 15 different structures studied, four, 4,12,2-, sR-, γ-, and 6,6,12-graphynes, show promising potential for CRR. B-doped 4,12,2-graphyne, with small square and rectangular pores, had a very low C-C coupling barrier of 0.34 eV with strong 2*CO adsorption (-2.5 eV). Furthermore, our research revealed a correlation between the heat of reaction (2*CO → *OCCO) and the average pore area around the acetylenic linker reaction site. Smaller pores give a favorable orientation of two CO molecules, resulting in low reaction barriers.
Poobodin Mano, Supawadee Namuangruk, Kaito Takahashi
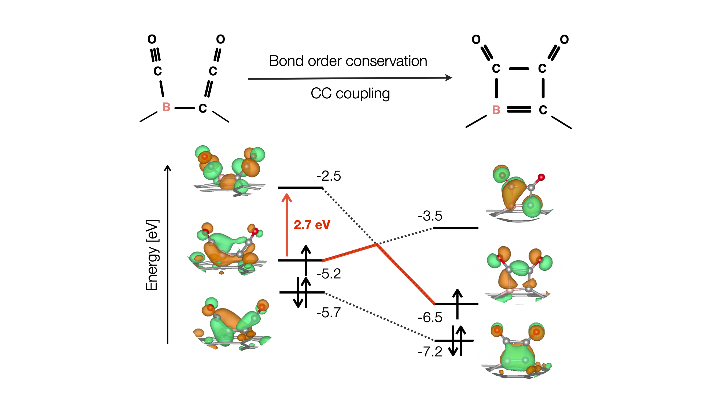
For CO2 electroreduction to produce C2+ products, many non-metal catalyst studies have focused on doping to sp2 and sp3 carbon material for efficient Carbon-Carbon (CC) bond formation. However, studies on doping to sp hybridization are scarce, so we focused on graphyne (GRY), a stable carbon allotrope with sp-sp2 hybridization. We theoretically showed that ethanol could be selectively produced with a low limiting potential of -0.53 V with a very low CC coupling barrier of 0.46 eV by boron doping (B-GRY) to the highly abundant sp hybrid linker. Fascinatingly, strong CO binding energy coexisted with a low CC coupling barrier, thus breaking the Bell-Evans-Polanyi relation. This provides a new direction that differs from previous studies that utilized the weakening of CO binding energy to reduce the CC coupling barrier. Our calculations using an integrated crystal orbital bond index showed that for 2*CO, the binding with B-GRY includes both s donation and π back donation, while only s binding with B-GRY occurs for *COCO. The electrons responsible for the π back donation for CO binding were used for the OC-CO bond formation after CC coupling. The reactive π electron on the sp hybrid linker has a chameleon-like feature changing its orbital hybridization to donate electrons depending on the adsorbate. We utilized the integrated crystal orbital bond index along the ethanol production pathway for the first time and showed that the bond order conservation effectively promotes CC coupling on B-GRY.
Jake Tan and Kaito Takahashi
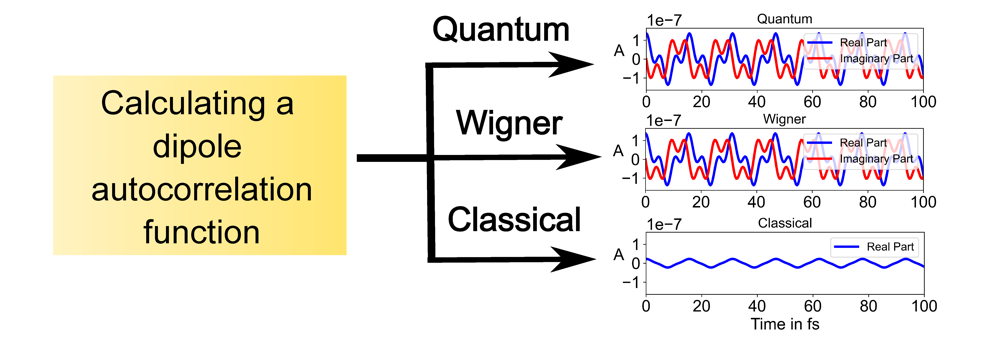
Infrared spectra have been used in many chemical applications, and theoretical calculations have been useful for analyzing these experimental results. While quantum mechanics is used for calculating the spectra for small molecules, classical mechanics is used for larger systems. However, a systematic understanding of the similarities and differences between the two approaches is not clear. Previous studies focused on peak position and relative intensities of the spectra obtained by various quantum and classical methods, but here, we included “absolute” intensities in the evaluation. The infrared spectrum of a one-dimensional (1D) harmonic oscillator and Morse oscillator were examined using four treatments: quantum, Wigner, truncated-Wigner, and classical microcanonical treatments. For a 1D harmonic oscillator with a linear dipole moment function (DMF), the quantum and Wigner treatments give nearly the same spectra. On the other hand, the truncated-Wigner underestimates the fundamental transition’s intensity by half. In the case of cubic DMF, the truncated-Wigner and classical methods fail to reproduce the relative intensity between the fundamental and second overtone transitions. Unfortunately, all the Wigner and classical methods fail to agree with the quantum results for a Morse oscillator with just 1% anharmonicity.
Yen-Hsiu Lin Kaito Takahashi* and Jim Jr-Min Lin*
Physical Chemistry Chemical Physics 24, 10439- (2022)
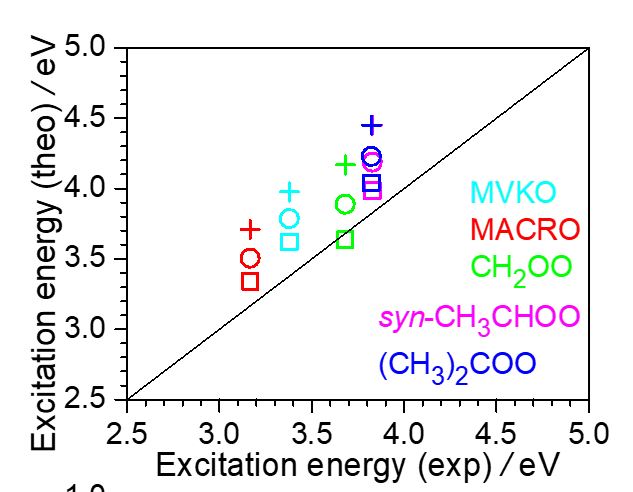
Methyl vinyl ketone oxide (MVKO) and methacrolein oxide (MACRO) are resonance-stabilized Criegee intermediates which are formed in the ozonolysis reaction of isoprene, the most abundant unsaturated hydrocarbon in the atmosphere. The absolute photodissociation cross sections of MVKO and MACRO were determined by measuring their laser depletion fraction at 352 nm, which was deduced from their time-resolved UV-visible absorption spectra. After calibrating the 352-nm laser fluence with the photodissociation of NO2, for which the absorption cross section and photodissociation quantum yield are well known, the photodissociation cross sections of thermalized (299 K) MVKO and MACRO at 352 nm were determined to be (3.02 ± 0.60) × 10−17 cm2 and (1.53 ± 0.29) × 10−17 cm2, respectively. Using their reported spectra and photodissociation quantum yields, their peak absorption cross sections were deduced to be (3.70 ± 0.74) × 10−17 cm2 (at 371 nm, MVKO) and (3.04 ± 0.58) × 10−17 cm2 (at 397 nm, MACRO). These values agree fairly with our theoretical predictions and are substantially larger than those of smaller, alkyl-substituted Criegee intermediates (CH2OO, syn-CH3CHOO, (CH3)2COO), revealing the effect of extended conjugation. With their cross sections, we also quantified the synthesis yields of MVKO and MACRO in the present experiment to be 0.22 ± 0.10 (at 299 K and 30 – 700 Torr) and 0.043 ± 0.019 (at 299 K and 500 Torr), respectively, relative to their photolyzed precursors. The lower yield of MACRO can be related to the high endothermicity of its formation channel.
 中央研究院 原子與分子科學研究所
中央研究院 原子與分子科學研究所

