Yi-Shan Wu, Li-Kang Chu,* Tsyr-Yan Yu*
J Phys Chem Lett .

Coccolithophores play critical roles in global carbon and sulfur cycles. They contribute to the carbon cycle through photosynthesis and calcification and the sulfur cycle by producing dimethylsulfoniopropionate (DMSP). Despite their ecological importance, the details and dynamics of methionine metabolism in coccolithophores are poorly understood. Here, we introduce an in situ light-coupled nuclear magnetic resonance (NMR) spectroscopy setup to monitor methionine metabolism directly in coccolithophore cultures under varying environmental conditions. Combining in situ light-coupled NMR spectroscopy and 13C magic angle spinning (MAS) spectroscopy, we observed that coccolithophores can take up methionine and convert it into 4-methylthio-2-oxobutyrate (MTOB), which is subsequently secreted into the culture medium, while DMSP was detected only intracellularly. Furthermore, environmental factors, such as elevated temperatures at 24.8 °C, which is 6.8 °C higher than the typical growth temperature for coccolithophores, and darkness, accelerated methionine consumption but reduced its incorporation into proteins and its conversion into MTOB, suggesting a shift toward alternative metabolic pathways under stress. In contrast, seawater acidification had minimal effects on the methionine metabolism. These findings provide new insights into how environmental conditions influence sulfur metabolism in coccolithophores, with potential consequences for their ecological functioning under future climate scenarios.
Yu-Chen Feng, Sashank Agrawal, Chin-Hao Yang, Hao-ChihChang, Ling Kuo, Wen-Chung Yu, Yo-Tsen Liu,* and Tsyr-YanYu*
Chemistry - An Asian Journal

Transthyretin (TTR), a homo-tetrameric protein encoded bythe TTR gene, can lead to amyloid diseases when destabilized bymutations. The TTR-Ala97Ser (A97S) mutation is the predominantpathogenic variant found in Han-Taiwanese patients and isassociated with late-onset familial amyloid polyneuropathy (FAP),which presents a rapid progression of symptoms affecting peripheralnerves and the heart. In this study, we combined nuclear magneticresonance (NMR) spectroscopy and X-ray crystallography toinvestigate how the A97S mutation impacts the structure anddynamics of TTR. Previous X-ray analyses indicated that the FG loopexhibits increased flexibility due to the mutation, evidenced by missingelectron density and a reduced number of hydrogen bonds. Our NMRhydrogen-deuterium (H/D) exchange experiments provided additionalinsights, revealing that inter-residue hydrogen bonds among the FGloop residues are unstable in both wild-type (WT) and A97S TTR.Notably, the hydrogen bonds between G67 and S97 are unstable,influencing the stability of adjacent loops. This elongation of the FGloop is believed to contribute to increased flexibility and enhancedwater-protein proton exchange, as observed in NMR relaxation andc hemical exchange experiments. Our findings offer a comprehensiveunderstanding of how the A97S mutation affects TTR structure anddynamics, providing new insights into its amyloidogenicity
Hsu Cheng-Hsun, Yu Hsin-Yu, Lee Ho Jun, Wu Pei-Hao, Huang Shing-Jong, Lee Jong Suk*, Yu Tsyr-Yan*, Li Yi-Pei*, Kang Dun-Yen*
Angewandte Chemie International Edition

Water and other small molecules frequently coordinate within metal-organicframeworks(MOFs). These coordinated molecules may actively engage in mass transfer, moving together with the transport molecules, but this phenomenon has yet to be examined. In this study, we explore a unique water transfer mechanism in UTSA-280, where an incoming water molecule can displace a coordinated molecule for mass transfer. We refer to this process as the“knock-off”mechanism. Despite UTSA-280 possessing one-dimensional channels, the knock-off transport enables water movement along the other two axes, effectively simulating a pseudo-three-dimensional mass transfer. Even with a relatively narrow pore width, the knock-off mechanism enables a high water flux in the UTSA-280 membrane.The knock-off mechanism also renders UTSA-280 superior water/ethanol diffusion selectivity for per vaporation.To validate this unique mechanism, we conducted 1H and 2H solid-state NMR on UTSA-280 after the adsorption of deuterated water. We also derived potential energy diagrams from the density functional theory to gain atomic-level insight into the knock-off and the direct-hopping mechanisms.The simulation findings reveal that the energy barrier of the knock-off mechanism is marginally lower than the direct-hopping pathway, implying its potential role in enhancing water diffusion in UTSA-280.
Cheng-Chieh Lin, Shing-Jong Huang, Pei-Hao Wu, Tzu-Pei Chen, Chih-Ying Huang, Ying-Chiao Wang, Po-Tuan Chen, Denitsa Radeva, Ognyan Petrov, Vladimir Gelev, Raman Sankar, Chia-Chun Chen,
Chun-Wei Chen*, Tsyr-Yan Yu*
Nature Communications, 13, 1513 (2022).
Limited methods are available for investigating the reorientational dynamics of A-site cations in two-dimensional organic–inorganic hybrid perovskites (2D OIHPs), which play a pivotal role in determining their physical properties. Here, we describe an approach to study the dynamics of A-site cations using solid-state NMR and stable isotope labelling. 2H NMR of 2D OIHPs incorporating methyl-d3-ammonium cations (d3-MA) reveals the existence of multiple modes of reorientational motions of MA. Rotational-echo double resonance (REDOR) NMR of 2D OIHPs incorporating 15N- and 13C-labeled methylammonium cations (13C,15N-MA) reflects the averaged dipolar coupling between the C and N nuclei undergoing different modes of motions. Our study reveals the interplay between the A-site cation dynamics and the structural rigidity of the organic spacers, so providing a molecular-level insight into the design of 2D OIHPs.
Peibin Zhong,# Chun Hao Liu,# Yit-Tsong Chen* and Tsyr-Yan Yu*
ACS Appl. Bio Mater. 2020, 3, 9, 6351–6357; DOI: 10.1021/acsabm.0c00783
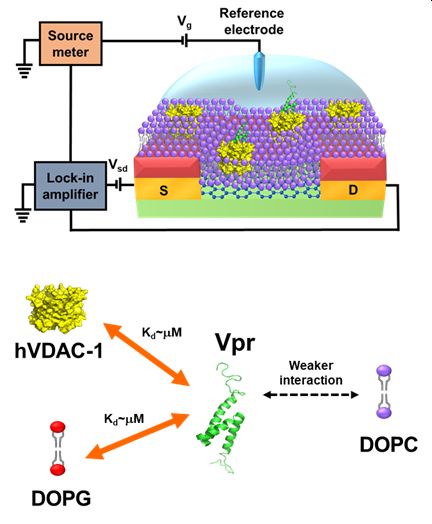
The viral protein R (Vpr) of human immunodeficiency virus 1 (HIV-1) is involved in many cellular processes during the viral life cycle; however, its associated mechanisms remain unclear. Here, we designed an E. coli expression construct to achieve a milligram yield of recombinant Vpr. In addition, we fabricated a graphene field-effect transistor (G-FET) biosensor, with the modification of a supported lipid bilayer (SLB), to study the interaction between Vpr and its interaction partners. The Dirac point of the SLB/G-FET was observed to shift in response to the binding of Vpr to SLB. By fitting the normalized shift of the Dirac point as a function of Vpr concentration to the Langmuir adsorption isotherm equation, we could extract the dissociation constant (Kd) to quantify the Vpr-binding affinity. When 1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (DOPG) mem-brane was used as the SLB, the dissociation constant was determined to be 9.6 ± 2.1 μM. In contrast, only a slight shift of the Dirac point was observed in response to the addition of Vpr when 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) membrane was used as the SLB. Taking the advantage of the much weaker binding of Vpr to DOPC membrane, we prepared human voltage-dependent anion channel isoform 1 (hVDAC-1) embed-ded DOPC membrane as the SLB for G-FET, and used it to determine the dissociation constant as 5.1 ± 0.9 μM. In summary, using clinically relevant Vpr protein as an example, we demonstrated that a SLB/G-FET bio-sensor is a suitable tool for studying the interaction between a membrane associated protein and its interac-tion partners.
Yo-Tsen Liu, Yueh-Jung Yen, Frans Ricardo, Yu Chang, Pei-Hao Wu, Shing-Jong Huang, Kon-Ping Lin*,Tsyr-Yan Yu*
Annals of Clinical and Translational Neurology, 2019 Oct;6(10):1961-1970. DOI:10.1002/acn3.50887
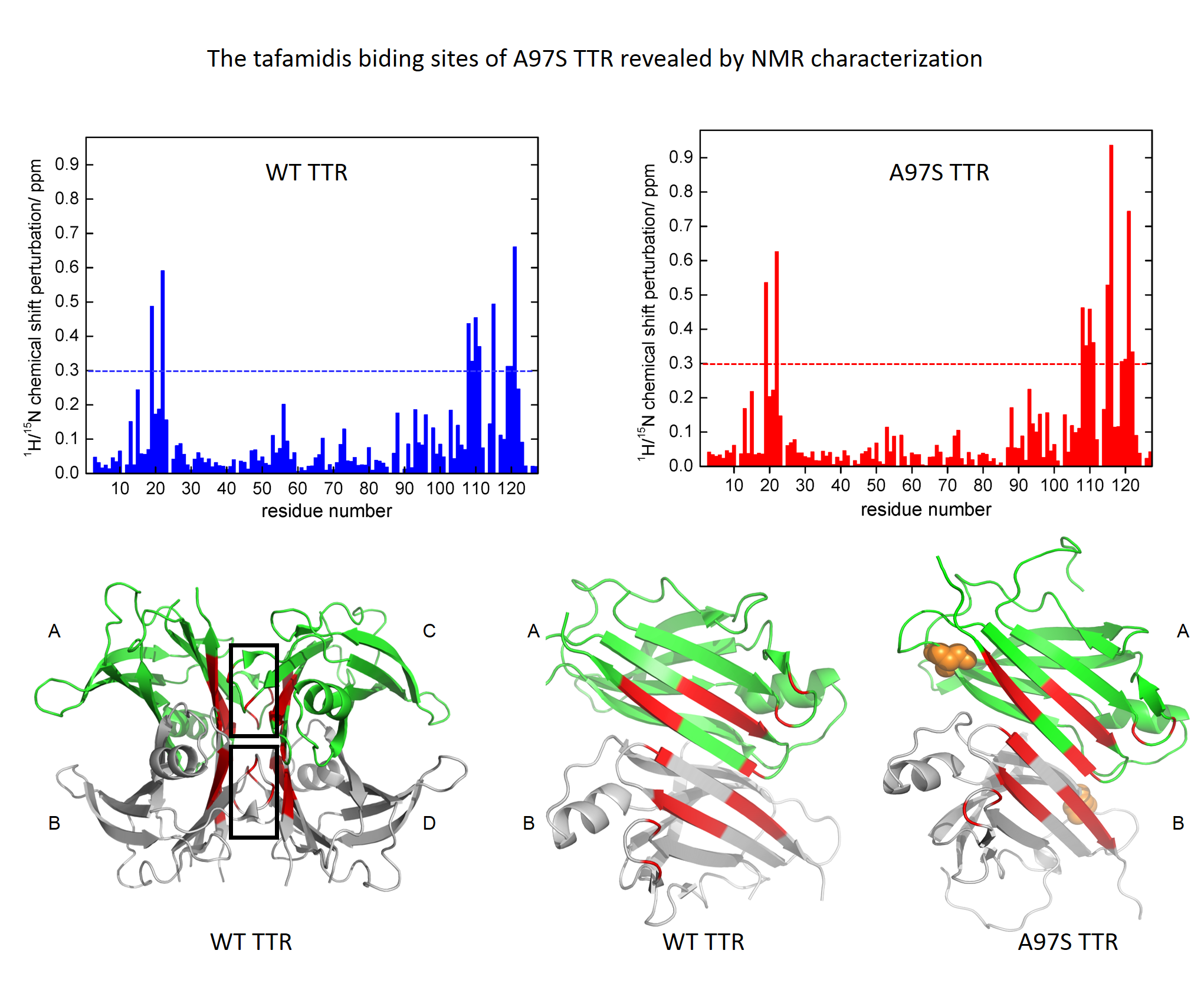
Objective: Ala97Ser (A97S) is the major transthyretin (TTR) mutation in Taiwanese patients of familial amyloid polyneuropathy (FAP), characterized by a late-onset but rapidly deteriorated neuropathy. Tafamidis can restore the stability of some mutant TTR tetramers and slow down the progression of TTR-FAP. However, there is little understanding of the biophysical features of A97S-TTR mutant and the pharmacological modulation effect of tafamidis on it. This study aims to delineate the biophysical characteristics of A97S-TTR and the pharmacological modulation effect of tafamidis on this mutant.
Method: The stability of TTR tetramers was assessed by urea denaturation and differential scanning calorimetry. Isothermal titration calorimetry (ITC) was used to measure the binding constant of tafamidis to TTR. Nuclear magnetic resonance spectroscopy (NMR) titration experiment was used to map out the tafamidis binding site.
Results: Chemical and thermal denaturation confirmed the destabilization effect of A97S. In consistent with other amyloidogenic mutant, A97S-TTR has slightly lower conformational stability. NMR revealed the binding site of A97S-TTR with tafamidis is at the thyroxine binding pocket. The ITC experiments documented the high affinity of the binding which can effectively stabilize the A97S-TTR tetramer.
Interpretation: This study confirmed the structural modulation effect of tafamidis on A97S-TTR and implied the potential therapeutic benefit of tafamidis for A97S TTR-FAP. This approach can be applied to investigate the modulation effect of tafamidis on other rare TTR variants and help to make individualized choices of available treatments for FAP patients.
